Glatiramer Acetate (GA)/Copaxone API Sameness Models
Hooke has extensive experience running studies to establish active pharmaceutical ingredient (API) sameness of generic GA and Teva Copaxone, as well as developing models for that purpose.
The FDA recommends that generic GA sponsors conduct testing in at least two EAE assays (one prophylactic and one therapeutic). Hooke routinely performs these assays.
Sameness models
Hooke recommends three models of experimental autoimmune encephalomyelitis (EAE) for assessment of generic GA sameness:
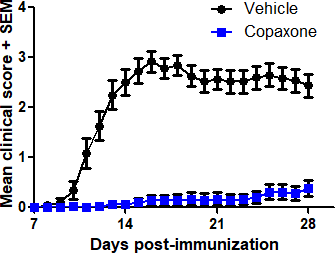
- MOG35-55/CFA-induced EAE (prophylactic treatment)
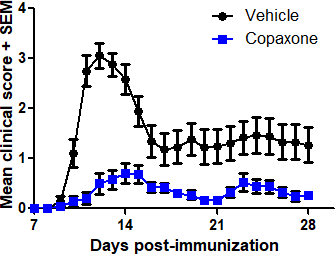
- [Ser140]-PLP139-151/CFA-induced EAE (prophylactic treatment)
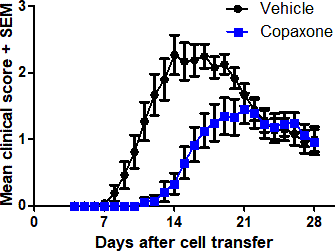
- Adoptive transfer EAE (therapeutic treatment)
In each model, mice are treated with the Reference Listed Drug (RLD) Teva Copaxone, the generic GA test compound, or vehicle, and clinical symptoms of EAE are monitored for 28 days.
Typical results
Results from studies conducted at Hooke in each of the three models are shown below.
Please contact Hooke at or with questions or for a quotation.


_150px.jpg)