Adoptive Transfer EAE
Hooke runs most commonly-used EAE models. Click here for an overview of EAE and a list of models offered.
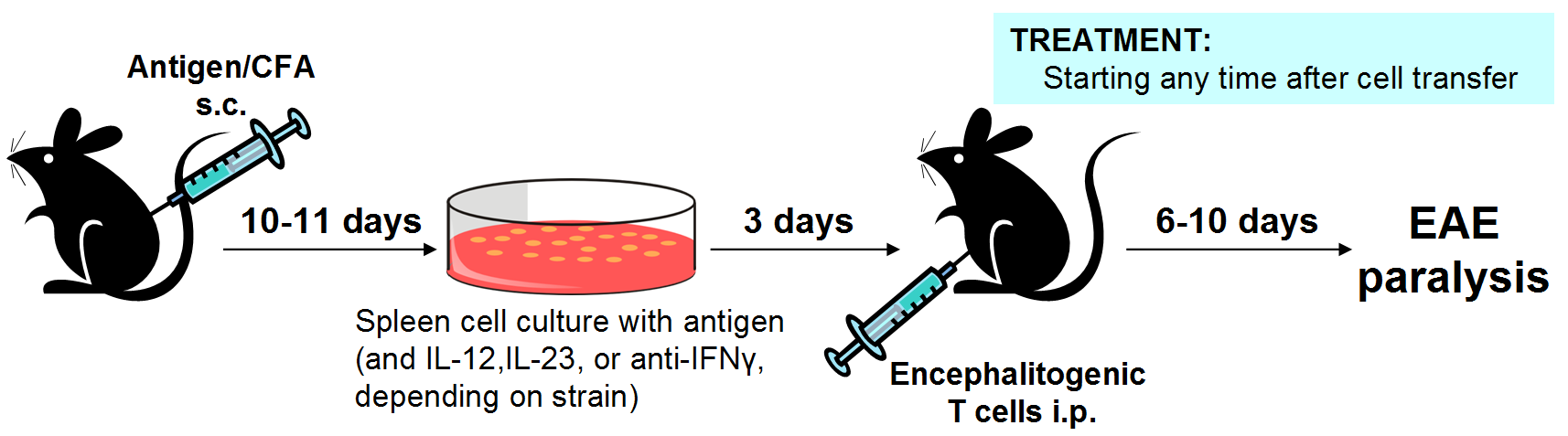
In the adoptive transfer model, donor mice are immunized to generate encephalitogenic T cells, which are then activated in cell culture and transferred into recipient mice to induce EAE. Treatment is usually administered only to recipient mice, but can be administered to donor mice or added to encephalitogenic cells during culture.
Because fully encephalitogenic T cells are transferred into the recipient mice, this is considered a model of therapeutic treatment.
This model is very robust and more sensitive than other therapeutic EAE models.
Adoptive transfer EAE separates the induction phase of EAE from the effector phase. In "direct" EAE models the induction phase (when encephalitogenic cells are generated in response to immunization) continues during the effector phase (because of ongoing priming). This makes it impossible to isolate the phases. In adoptive transfer EAE, the induction phase occurs in donor mice, while the effector phase occurs in the recipient mice, isolating the phases.
Cell trafficking can be studied in this model by using congenic strains of donor and recipient mice; flow cytometric analysis can distinguish markers unique to the injected cells.
Compounds targeting Th1 or Th17 effects can be studied by manipulation of cell culture conditions to cause either cell type to dominate the population of transferred cells.
The model is 5 weeks long and requires 10 to 12 mice/group for good statistical results. The model can be run in either C57BL/6 or SJL mice; donor and recipient mice are the same strain except when using congenic strains to study cell trafficking in vivo.
FTY720 (fingolimod, Gilenya) and anti-VLA-4 antibodies are the most commonly used positive controls.
Please contact Hooke at or with questions or for a quotation.
Typical results in this model
| Treatment | EAE incidence | MMS ± SEM |
p value (MMS) |
End score ± SEM |
p value (end score) |
End weight (%) ± SEM |
p value (end weight) |
|---|---|---|---|---|---|---|---|
| Vehicle | 100% | 3.20 ± 0.35 | 2.55 ± 0.37 | 84.8 ± 5.80 | |||
| Anti-VLA-4 | 90% | 1.65 ± 0.97 | 0.001 | 1.55 ± 1.010 | 0.017 | 97.3 ± 10.3 | 0.004 |
| FTY720 | 100% | 2.35 ± 0.41 | 0.001 | 1.95 ± 0.44 | 0.008 | 91.5 ± 8.73 | 0.059 |
SJL mice vs C57BL/6 mice
Hooke runs adoptive transfer EAE in both SJL and C57BL/6 mice. Except when using congenic mice, the same mouse strain is used for both donors and recipients. The model is similar in either strain.
In SJL mice, culture conditions can be modified to result in Th1 or Th17 dominance.
In C57BL/6 mice it appears that both Th1 and Th17 cells are required for EAE induction. C57BL/6 mice can be used to study cell trafficking. Using B6.PL-Thy1a/CyJ or B6.SJL-Ptprca Pep3b/BoyJ mice as donors and C57BL/6 mice as recipients (congenic strains), encephalitogenic T cells can be tracked in vivo in the recipient mice.
Disease induction and development
Donor mice are immunized to generate encephalitogenic helper T cells (CD4+) specific for myelin-derived antigens ([Ser140]-PLP139-151 for SJL mice, MOG35-55 for C57BL/6 mice). These cells are then activated in cell culture and injected into recipient mice, where EAE develops.
When using SJL mice, Th1 or Th17 cells can be preferentially generated by adding IL-12, IL-23, or anti-IFNγ to the cell culture.
Because fully encephalitogenic T cells are transferred into the recipient mice, this model is considered therapeutic even if recipient mice receive treatment starting on the day of cell injection (6-10 days before disease onset).
Adoptive transfer EAE induction
Tracking encephalitogenic cells in vivo
By using B6.PL-Thy1a/CyJ or B6.SJL-Ptprca Pep3b/BoyJ mice as donors and C57BL/6 mice as recipients, this model can be used to track encephalitogenic T cells in vivo in recipient mice.
These mouse strains are congenic. T cells from B6.PL-Thy1a/CyJ mice express the Thy1a (Thy1.1) marker, while T cells from C57BL/6 mice express Thy1b (Thy1.2). T cells from B6.SJL-Ptprca Pep3b/BoyJ mice express CD45.1, vs. the CD45.2 marker in C57BL/6 mice. Donor T cells can therefore be distinguished from recipient T cells using flow cytometry.
Intracellular staining for cytokines can reveal changes in cytokine production.
Tissue collection and end-of-study analysis
Hooke offers an extensive set of tissue collection and analysis options. Click here for more information.


_150px.jpg)