Systemic Lupus Erythematosus (SLE) in MRL/lpr Mice
We routinely run systemic lupus erythematosus (SLE) in MRL/lpr mice at Hooke. Because it clinically resembles SLE in human patients and is relatively short (10 weeks), this is the most commonly used model of SLE.
MRL/lpr mice are homozygous for spontaneous mutation in the Fas gene, and develop lymphadenopathy (enlarged lymph nodes), splenomegaly (enlarged spleen), anti-nuclear and anti-dsDNA antibodies, and systemic autoimmunity. Autoimmune responses occur spontaneously in these mice, resulting in glomerulonephritis and arthritis resembling SLE in human patients.
We run this model in female mice; treatment usually starts when mice are 11 weeks old. By 19 weeks of age, ~85% of untreated mice will develop kidney pathology - usually studies end at that point.
Our standard in vivo readouts are proteinuria and body weight, each measured weekly.
At the end of the study, we measure spleen weight, perform histological analysis of kidneys, and measure anti-dsDNA antibodies and blood urea nitrogen (BUN) in serum. We can also perform immunofluorescence analysis of kidneys and evaluate IgG and C3 presence in the kidney cortex (glomeruli). Spleens can be evaluated histologically to determine the follicle area as a proportion of the spleen area.
Autoimmune responses such as production of anti-nuclear antibodies are detectable as early as 8 weeks of age. Extensive kidney pathology is found at 16 to 30 weeks of age.
Please contact Hooke at or with questions or for a quotation.
Typical results
Mice were treated from week 11 of life (Week 11) through the end of the study (Week 19). Proteinuria was measured once weekly from Week 10. Serum was collected at Weeks 11 and 16 for anti-dsDNA IgG antibody measurement, and at Week 19 for anti-dsDNA IgG antibody and BUN measurement. Kidneys were collected at the end of the study for histological analysis.
All figures show mean + or ± SEM, and all tables show mean ± SD.
Clinical readouts
| Treatment | # mice | End proteinuria score | p value |
|---|---|---|---|
| Vehicle | 13* | 3.38 ± 0.77 | - |
| Cyclophosphamide | 10 | 2.25 ± 0.42 | <0.001 |
*Two (2) mice were euthanized due to complications of lymphadenopathy.
End proteinuria scores compared using Wilcoxon's non-parametric test.
Tissue weights at termination
| Treatment | Kidney (mg)* | p value | Spleen (mg) | p value |
|---|---|---|---|---|
| Vehicle | 233 ± 51 | - | 496 ± 249 | - |
| Cyclophosphamide | 191 ± 32 | 0.036 | 116 ± 37 | <0.001 |
Kidney and spleen weights compared using 2-tailed Student's t-test.
*Average of both kidneys.
BUN and anti-dsDNA measurements
| Treatment | BUN in serum (mg/dL) | p value |
|---|---|---|
| Vehicle | 58.4 ± 76.8 | - |
| Cyclophosphamide | 11.0 ± 5.0 | 0.066 |
BUN in serum concentrations compared using 2-tailed Student's t-test.
| anti-dsDNA IgG (units/mL) | |||||
|---|---|---|---|---|---|
| Treatment | Week 21 (baseline) |
Week 16 | p value | Week 19 (terminal) |
p value |
| Vehicle | 68 ± 69 | 147 ± 142 | - | 183 ± 135 | - |
| Cyclophosphamide | 28 ± 18 | 0.016 | 23 ± 6 | 0.001 | |
Anti-dsDNA antibody concentrations compared using 2-tailed Student's t-test.
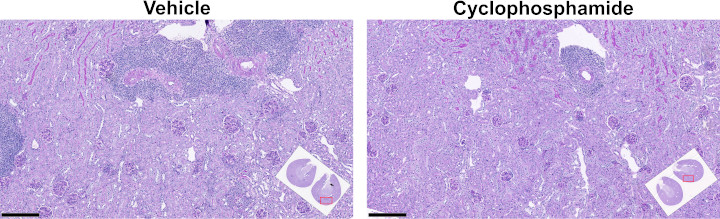
Histological analysis of kidneys at termination
| Histological analysis of kidneys at termination | ||||||
|---|---|---|---|---|---|---|
| Treatment | Total glomerular lesion | p value | Total tubular and interstitial lesion | p value | Total kidney lesion | p value |
| Vehicle | 5.2 ± 3.7 | - | 2.2 ± 1.8 | - | 7.4 ± 5.0 | - |
| Cyclophosphamide | 0.3 ± 0.7 | <0.001 | 0.3 ± 0.5 | <0.001 | 0.6 ± 0.8 | <0.001 |
Kidney lesion scores compared using Wilcoxon's non-parametric test.
For a higher resolution image, click on the image above. Scale bars are 250 µm. Insets illustrate kidney transverse sections at the hilus (kidney cut in half, both halves embedded and sectioned), with red outlines indicating the locations of the magnified images.
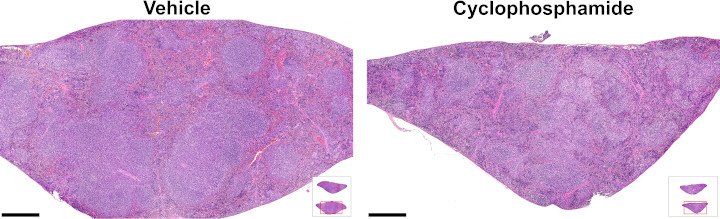
Histological analysis of spleens at termination
Mice were treated from Week 11 through Week 19. Spleens were collected for histological analysis at the end of the study (Week 19).
| Histological analysis of spleens at termination | |||
|---|---|---|---|
| Treatment | n | Follicle area of tissue area (%) | p value |
| Vehicle | 15 | 71.5 ± 8.2 | - |
| Cyclophosphamide | 15 | 61.1 ± 11.1 | 0.009 |
Follicle areas of tissue areas compared using 2-tailed Student's t-test.
For a higher resolution image, click on the image above. Scale bars are 500 µm. Insets illustrate transverse sections of spleens (spleen cut in half, both halves embedded and sectioned) with red outlines indicating the locations of the magnified images.


_150px.jpg)