Imiquimod-Induced Psoriasis in C57BL/6 Mice
In this model, mice develop skin inflammation characterized by redness, skin thickening, and plaque development similar to human psoriasis.
Psoriasis
Psoriasis is common chronic inflammatory disease of the skin of unknown cause. It is characterized by increased proliferation and lack of maturation of keratinocytes, resulting in thickened skin. Dermis and epidermis are infiltrated with immune cells which, together with keratinocytes, produce proinflammatory cytokines (IL-1β, IL-6, TNF-α, IL-23, IL-22, IL-17A and IL-17F) and keep inflammatory processes in psoriatic lesions active.
Approved treatments for psoriasis include:
- Corticosteroids
- TNF blockers: adalimumab (HUMIRA, Cyltezo); etanercept (Enbrel, Erelzi); infliximab (Remicade)
- IL-17A blockers: ixekizumab (Taltz); brodalumab (Siliq); secukinumab (Cosentyx)
- IL-23 blockers: guselkumab (Tremfya); ustekinumab (Stelara); tildrakizumab (Ilumya)
- PDE4 inhibitor: apremilast (Otezla)
Imiquimod-induced psoriasis model
This model is usually run in female C57BL/6 mice. Topical application of imiquimod induces cellular infiltration, cytokine responses, epidermal thickening, scaling, and erythema analogous to human plaque-type psoriasis. In C57BL/6 mice, this response includes induction of cytokines IL-17A, IL-23, and IL-22.
To induce disease, imiquimod is applied to the ear and back of mice daily for 5 days (Days 0 to 4).
Treatment may be prophylactic, or therapeutic, usually starting 2 days after initial imiquimod application.
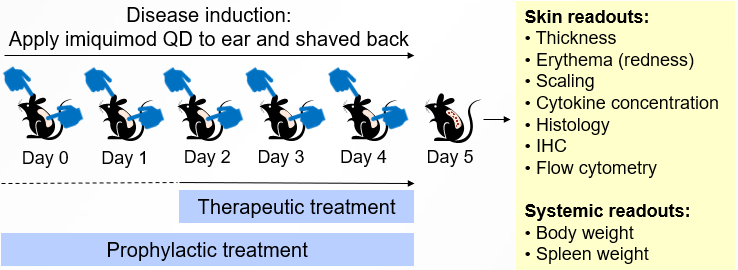
Our standard in vivo readouts are mouse weight, ear and skin thickness, and Psoriasis Area and Severity Index (PASI) scoring (skin color, thickness, and scaling).
At the end of the study, we typically perform cytokine analysis (in ear homogenates), measure spleen weight, and run histological analysis of skin. Flow cytometric analysis of skin-infiltrating cells can also be performed.
Scoring
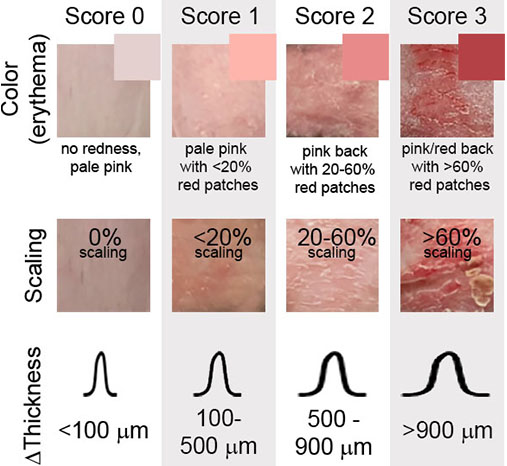
Psoriasis is scored using the Psoriasis Area Severity Index (PASI), which is based on skin color (erythema), skin scaling, and change in skin thickness from a measurement prior to imiquimod administration (healthy skin). Skin thickness is measured with calipers across a fold of skin.
Each of the three readouts are scored from 0 to 3 (see scoring guide below) and the scores are summed (to the range 0 to 9).
Typical results
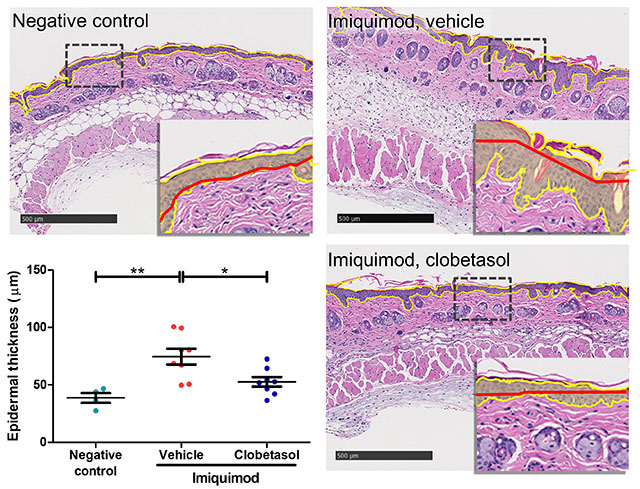
The results below are from a representative study at Hooke. Mice received either vehicle (negative control for disease) or 5% imiquimod (back and ear) QD for 5 days to induce disease. From Day 2 until sacrifice, mice that received imiquimod were dosed with vehicle (petroleum jelly) or clobetasol.
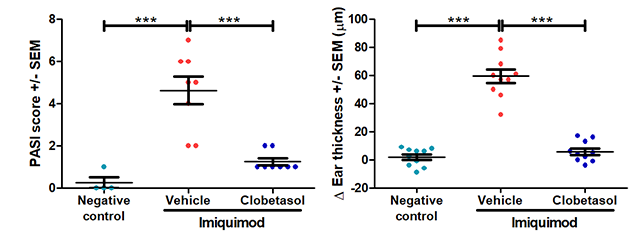
*** p<0.001, error bars are SEM. Statistical analysis used Student’s T-test comparing to vehicle/imiquimod control.
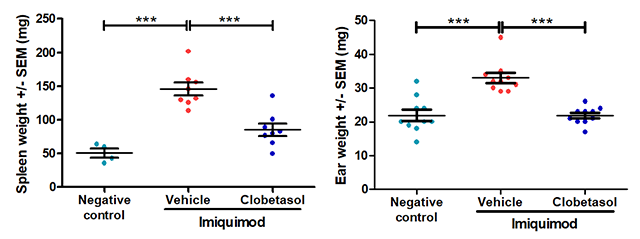
*** p<0.001, error bars are SEM. Statistical analysis used Student’s T-test comparing to vehicle/imiquimod control.
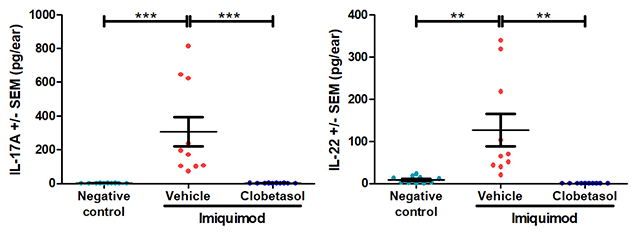
** p<0.01, *** p<0.001, error bars are SEM. Statistical analysis used Student’s T-test comparing to vehicle/imiquimod control.
Epidermal thickness was determined for each sample using an algorithm to determine the area (yellow border, yellow highlight [inset]) of the epidermis and divides it by the calculated epidermal length (red line) to give the epidermal thickness (width). Scale bars = 500 µm. * p<0.05, ** p<0.01, error bars are SEM. Statistical analysis used Student’s T-test comparing to vehicle/imiquimod control.
Please contact Hooke at or with questions or for a quotation.
References
Van der Fits L et al, J Immunol. 182(9):5836-5845 (2009)
Flutter B and Nestle FO, Eur J Immunol. 43:3138-3146 (2013)


_150px.jpg)