EAE Induction by Active Immunization in Lewis Rats
Recommended protocol for use with:
- Hooke Kit™ gpMBP69-88/CFA Emulsion (cat. no. EK-3110)
This kit is recommended for study of experimental autoimmune encephalomyelitis (EAE), including testing efficacy of potential therapeutics. It will induce EAE in Lewis rats by active immunization with antigen in an emulsion with complete Freund's adjuvant (CFA).
Each kit supplies enough material for 10 rats.
EAE onset for most rats occurs 10 to 12 days after immunization, with peak of disease usually 3 days after onset for each rat. The peak generally lasts 1 to 3 days. All rats will fully recover by Day 20 after immunization, without relapses.
Rats are typically observed for 3 weeks.
Rat selection and handling
For the most uniform EAE development, use female Lewis rats at age 10 to 14 weeks. All rats should be the same age. Male rats can be used and will develop slightly milder EAE.
We have observed clear differences in EAE development in rats (p < 0.05) in EAE development in rats obtained from different breeders; this can change over time. As of this writing (see version date at the end of this protocol), in the United States we recommend Lewis rats from Charles River Laboratories.
Successful induction of EAE requires low-stress rat handling and husbandry procedures, good injection technique, use of appropriate rats, and good quality, stable, antigen emulsion. Stress decreases EAE susceptibility; minimizing rat stress is very important for successful EAE induction.
For consistent EAE, minimize rat stress as follows:
- Acclimate rats to your lab for 7+ days before starting.
- Play with rats several times prior to immunization, to get them used to being handled.
- Follow recommended injection procedure (see details below).
- House rats in a quiet environment, without excessive noise or vibration.
- Try to do all procedures in the animal room. Avoid moving rats on carts.
- Anesthesia is not necessary. If anesthesia must be used per veterinary requirements, we recommend inhaled anesthesia to minimize stress.
Administration of antigen emulsion
Emulsion should be administered subcutaneously, at two sites, 0.1 mL/site (0.2 mL/rat total).
Good subcutaneous injection technique is important for successful EAE induction, both for proper emulsion administration and also to minimize animal stress. Because EAE susceptibility is influenced by stress, the same person should dose all groups in an experiment (because different people may inflict different amounts of stress).
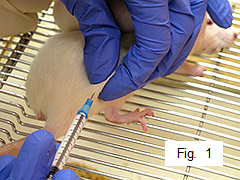
Have assistant gently restrain rat against cage.
Inject rat subcutaneously on one side of lower back with 0.1 mL of emulsion (Figure 1).
Keep the needle inserted into the subcutaneous space for 10 to 15 seconds to avoid leakage of the emulsion. Alternatively, a light pull on the syringe plunger will prevent leakage.
Inject rat subcutaneously on the other side of lower back with 0.1 mL of emulsion.
Again, keep the needle inserted into the subcutaneous space for 10 to 15 seconds or lightly pull on the syringe to avoid leakage.
Repeat (1-3) for all rats.
References
[1] McFarlin DE, Blank SE, Kibler RF, J. Immunol. 1 13:712 (1974)
[2] Kardys E and Hashim GA, J Immunol 127:862 (1981)
[3] Mannie MD, Paterson PY, U'Prichard DC, Flouret G., Proc Natl Acad Sci USA 82:5515 (1985)
[4] Hashim GA, Day ED, Fredane L, Intintola P, Carvalho E, J Neurosci Res. 16(3):467 (1986)
Appendix A - Rat EAE Scoring Guide
Typically, EAE is scored on scale 0 to 5. Most researchers also give rats "in-between" scores (i.e. 0.5, 1.5, 2.5, 3.5) when the clinical picture lies between two defined scores.
In most cases rats are scored daily, starting 7 days after immunization (Day 7) and continuing until Day 20.
We recommend the following scoring guidelines for rats:
Rat EAE scoring
| Score | Clinical observations |
|---|---|
| 0.0 | No obvious changes in motor function compared to non-immunized rats. When picked up, the tail has tension and some movement when finger runs along the tail. |
| 0.5 | Tip of tail is limp. When picked up, the tail has tension except for the tip. When walking, the tip of tail is dragged on the cage bottom. |
| 1.0 | Limp tail. When picked up, the whole tail is limp. The walk is not affected. |
| 1.5 | Limp tail and hind leg inhibition. When picked up, the whole tail is limp. Walking is very slightly wobbly. |
| 2.0 | Limp tail and weakness of hind legs. When picked up, legs are held close together. When the rat is observed walking, it has a clearly apparent wobbly walk. One foot may have toes dragging, but the other leg has no apparent inhibitions of movement. |
| 2.5 | Limp tail and dragging of hind legs. Both hind legs have some movement, but both are dragging at the feet (rat trips on hind feet). - OR - No movement in one leg/completely dragging one leg, but movement in the other leg. |
| 3.0 | Limp tail and complete paralysis of hind legs (most common). - OR - Limp tail and almost complete paralysis of hind legs. One or both hind legs are able to paddle, but neither hind leg is able to move forward of the hind hip. - OR - Limp tail with paralysis of one front and one hind leg. |
| 3.5 | Limp tail and complete paralysis of hind legs. Rat is moving around the cage, but when placed on its side, is unable to right itself. Hind legs are together on one side of body. |
| 4.0 | Limp tail, complete hind leg and partial front leg paralysis. Rat is minimally moving around the cage but appears alert and feeding. Euthanasia is recommended after the rat scores 4.0. When the rat is euthanized because of severe paralysis, a score of 5.0 is entered for that rat for the rest of the experiment. |
| 4.5 | Complete hind and partial front leg paralysis, no movement around the cage. Rat is not alert. Rat has minimal movement in the front legs. The rat barely responds to contact. Euthanasia is recommended. When the rat is euthanized because of severe paralysis, a score of 5.0 is entered for that rat for the rest of the experiment. |
| 5.0 |
Rat is found dead due to paralysis. - OR - Rat is euthanized due to severe paralysis. |
Version: 2023-06-04


_150px.jpg)